P07 - Thomas Stamminger
Spatiotemporal control of human cytomegalovirus gene silencing
- Human Cytomegalovirus
- Silencing factors
- Repressor occupancy of viral genome
- Transcriptional signature dictating silencing
- Visualization of viral genome silencing
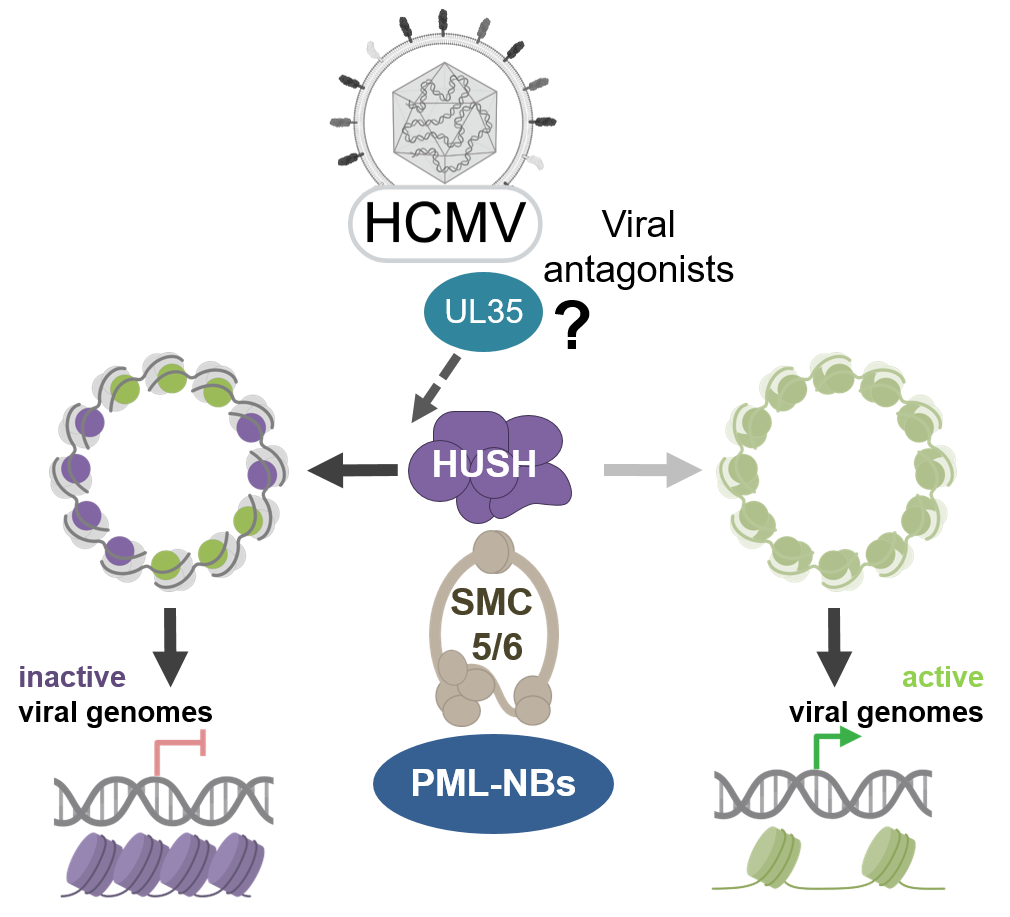
Human cytomegalovirus (HCMV), an extremely species-adapted herpesvirus, has developed a multitude of mechanisms to subvert host defenses ultimately resulting in lifelong viral persistence. Recent studies indicate a critical role of cell-intrinsic restriction machineries such as PML nuclear bodies (PML-NBs) for herpesvirus quiescence. Based on results obtained in a collaborative approach during the first funding period, we propose to study the role of two novel PML-associated repressor complexes, SMC5/6 and HUSH, for HCMV replication. The structural maintenance of chromosomes (SMC) 5/6 complex plays a pivotal role for DNA repair but also acts in an antiviral manner by compacting circular extrachromosomal DNA. For HUSH (Human Silencing Hub) two paralogous complexes have recently been described. HUSH1 is supposed to repress LINE-1 retrotransposons while HUSH2 preferentially targets interferon response genes. We propose a dual role of SMC5/6 and HUSH during HCMV infection. At the onset of HCMV transcription, these complexes may act in a repressive manner which needs to be antagonized by the virus. However, the prolonged replication cycle of HCMV may necessitate utilization of components of these machineries during later phases of infection. Consistent with antagonization of repression, we observed a strong upregulation of transposable elements (TEs) like LINE-1s very early during HCMV infection. Thus, the project has three main objectives: (i) to identify the viral proteins and mechanism(s) used to antagonize the repressive functions of HUSH and SMC5/6; (ii) to characterize functions of HUSH and SMC5/6 components needed during later phases of HCMV replication; (iii) to clarify the role of HUSH and other regulatory factors in the modulation of TEs during HCMV infection. We will study these protein complexes in a highly interactive and comparative manner together with other DEEP-DV members. This should provide new mechanistic insights into the role of these machineries for lytic replication and persistence of DNA viruses.


